half life formula chemistry
We use the equation A t 1 2 t t 1 2 A o where A t is the activity in time t A o is the original activity 500 1 2 10 t 1 2 6000 t 1 2. Half life formula By using the following decay formula the number of unstable nuclei in a radioactive element left after t can be calculated.

Half Life Expressions Chemistnate
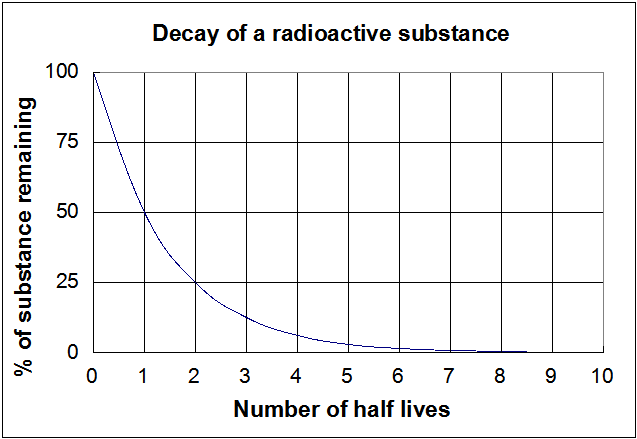
Graphical relations and half lives.
. N o Initial mass of the substance. Calculate the half-life of the radioactive source. Mean half life calculator uses the half life formula to compute results.
In other words exponential decay graphs have a. The half-life formula used to calculate zero order reaction is t₁₂ A₀2k. These practice questions will.
By putting these values in equation i we get. We know that at the half-life time eqt_12 eq the concentration of the reactant will be half as much as the initial concentration. T 12 is the half-life.
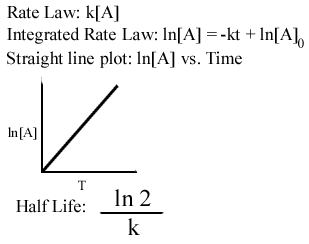
Therefore A t 1 2 A 0 at t 1 2. Ln 2 k t 12. Take a quick interactive quiz on the concepts in Half-Life Facts Formula Examples What is a Half-Life in Chemistry.
Or print the worksheet to practice offline. And for the second-order reaction the formula for the half-life of the reaction is given by 1k R 0. As in Figure 1 the graph in Figure 2 approaches the x-axis but will never reach it.
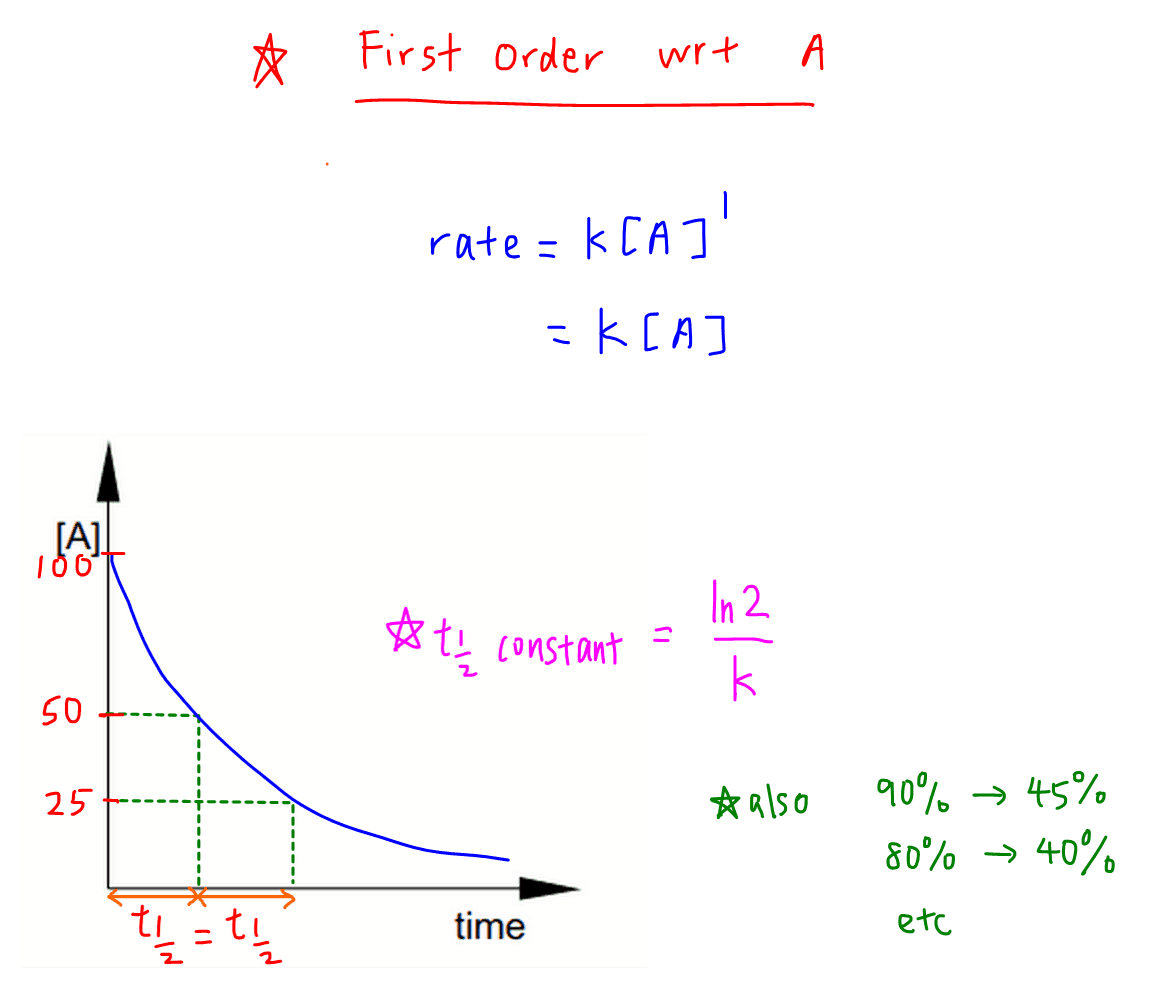
For the first-order reaction the half-life is defined as t12 0693k. Half life formula is. T 12 0693k.
For a second-order reaction the formula for the half-life of the reaction is. Where t12 is the half. On the x-axis T 12 T 1 2 is the symbol for half-life.
5 t T Nt N_0 times. Therefore we can set eqA eq equal to eqA_02. Here λ is called the disintegration or decay constant.
Hence the formula to calculate the half-life of a substance is. T 1 2 Half life of the. A We can calculate the half-life of the reaction using Equation ref2142.
K is the rate. The unit of half-life equation for zero order. N t Quantity os the substance remaining.
At half-life time t t 12 N N o 2. The half-life of a first-order reaction does not depend upon the. T Time elapsed.
The formula for the half-life is obtained by dividing 0693 by the constant λ. T ½ A o 2k For a first order reaction A products rate kA. For a first-order reaction the half-life is given by.
The half-life of a reaction is the time required for the reactant concentration to decrease to one-half its initial value. N t N 0 0. Half-life formula and unit for zero order reaction.
The half-life of a reaction t 1 2 is the time required for an initial reactant concentration A 0 to decrease by one-half. For a zero order reaction A products rate k. Half-life t 1 2 t log 2 log N o N t 2.
T ½ 0693 k For a. Ln N N o 2 k t 12. Half life is based around the decay process and the number of unstable nuclei remaining after time t.
Equations for Half Lives. Where t 12 is the half-life of the reaction. Where t 12 is the half-life time.
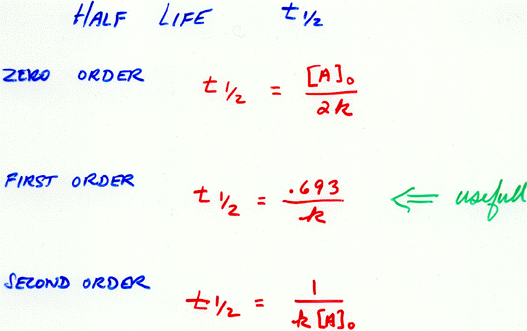
Chem 112 Lecture Spring 01 Overheads

Half Life And Carbon Dating Video Nuclei Khan Academy
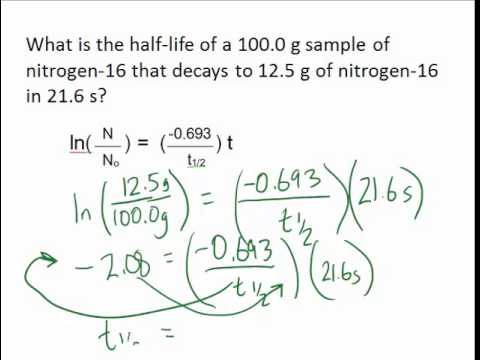
Chemistry Half Life Calculations Youtube

Identifying Half Life Given The Rate Constant Chemistry Study Com
Half Life Period Radioactive Decay Mean Life Byju S

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1
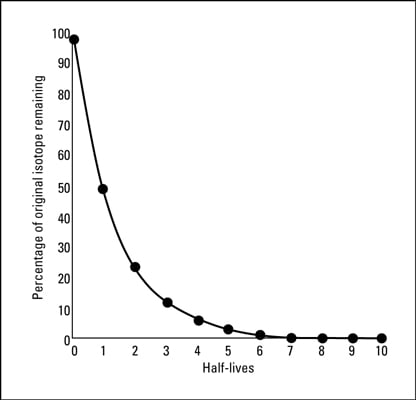
Nuclear Chemistry Half Lives And Radioactive Dating Dummies
Half Life Introductory Chemistry
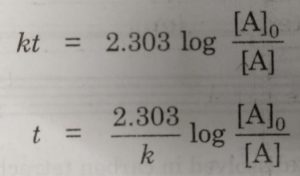
Half Life Period Of A Reaction Chemical Kinetics Chemistry Class 12
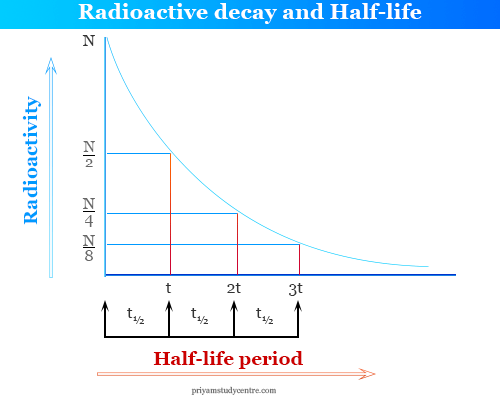
Radioactive Decay Half Life Definition Formula Calculation

Half Life Chemistry Problems Nuclear Radioactive Decay Calculations Practice Examples Youtube
Learn Chemistry Tutorials Kinetics Tutorial

How To Calculate Half Life Of A First Order Reaction Chemistry Study Com

Chemical Kinetics Chapter 14 Summary Of The Kinetics Reactions Orderrate Law Concentration Time Equation Half Life Rate K Rate K A Rate Ppt Download